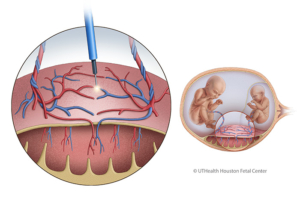
- UTHealth Houston Fetal Center
- Twin-to-twin transfusion syndrome
Twin-to-twin transfusion syndrome (TTTS): A comprehensive overview
What is TTTS?
Twin-to-twin transfusion syndrome (TTTS) is a rare but serious complication that can arise in utero in pregnancies involving identical twins who share a single placenta (monochorionic twins). Occurring in 10%-15% of such pregnancies, TTTS results from abnormal blood flow between the twins due to blood vessel connections on the shared placenta. One twin, referred to as the “donor,” transfers blood to the other twin, known as the “recipient.” This unequal distribution of blood creates significant challenges for both twins, often jeopardizing their health and survival.
An imbalance in blood flow
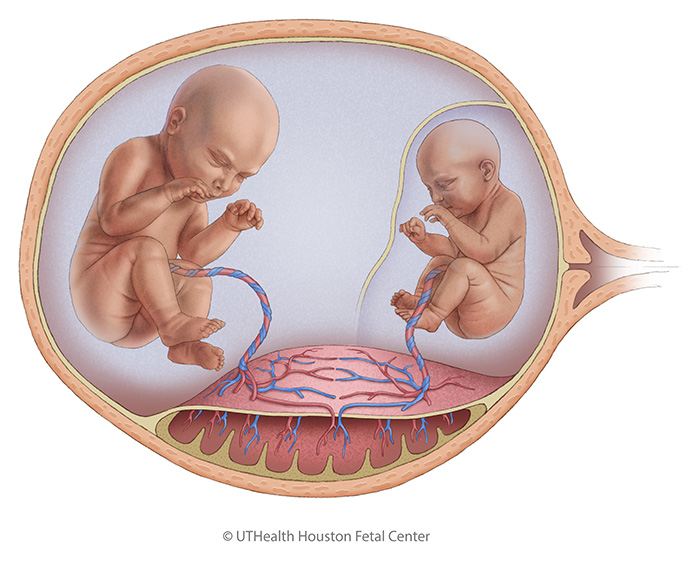
TTTS is caused by an imbalance in the blood exchange through shared placental blood vessels. The donor twin pumps blood to the recipient twin through these connections. This transfer leaves the donor twin with insufficient blood, leading to decreased urine production, low amniotic fluid levels (oligohydramnios), and a small or absent bladder. The recipient twin receives an excess of blood, causing overproduction of urine, high amniotic fluid levels (polyhydramnios), and a large bladder. This increased blood volume also places strain on the recipient’s heart, potentially resulting in fluid accumulation in more than one fetal cavity (hydrops), a form of heart failure.
There are no known genetic causes of TTTS, but the condition is unique to monochorionic pregnancies and highlights the importance of close monitoring during such pregnancies.
Symptoms and maternal impact
In addition to the risks posed to the twins, TTTS can significantly affect the mother. Excessive amniotic fluid from polyhydramnios may cause abdominal discomfort, shortness of breath, and an increased risk of premature labor or membrane rupture. Early diagnosis is crucial to prevent severe complications.
Diagnosing TTTS
The evaluation of TTTS begins with determining whether the twins share a placenta, typically via ultrasound during the first trimester. Key indicators of TTTS include:
- Same-gender twins
- Unequal twin size
- Discrepancies in the volume of amniotic fluid surrounding each twin
High-resolution ultrasound and fetal echocardiograms are essential for assessing the severity of the condition. Ultrasound helps identify structural abnormalities, placental location, and amniotic fluid levels. Fetal echocardiograms evaluate the impact of TTTS on the cardiovascular system, especially in the recipient twin, where heart strain is common. In some cases, genetic amniocentesis is performed to rule out other complications or anomalies.
It is vital to differentiate TTTS from other conditions, such as selective intrauterine growth restriction (sIUGR), which could have similar symptoms but may require different treatment.
Staging and monitoring
TTTS is a progressive disease. It is classified into five stages based on the severity of the condition. Stage 1 represents mild disease where intervention may not yet be necessary, while Stage 5 indicates the fetal demise of one or both twins. Monitoring typically includes regular ultrasounds to track both twins’ amniotic fluid levels, bladder size, and heart function.
Treatment options for TTTS
Treatment for TTTS varies depending on the stage and severity of the disease. Options include:
- Fetoscopic selective laser ablation
This minimally invasive procedure is the preferred treatment for moderate to severe TTTS. Using a fetoscope and laser, the shared blood vessels on the placenta are clotted to stop the abnormal blood exchange. This intervention improves survival rates, with at least one twin surviving in up to 90% of cases and both twins in 74%. Adverse neurological development occurs in around 14% of TTTS cases. - Selective cord occlusion
In severe cases where one twin is unlikely to survive, selective cord occlusion may be performed to protect the healthier twin. This procedure, though rarely used, prevents complications like neurological impairment in the surviving twin. - Expectant management
For mild cases (e.g., Stage 1), close monitoring with frequent ultrasounds is often sufficient to evaluate the progression of the disease. - Amnioreduction
Amnioreduction involves removing excess amniotic fluid from the recipient twin’s sac to reduce maternal discomfort and prolong the pregnancy. However, this is a temporary solution as fluid often reaccumulates, requiring repeated procedures.
Delivery considerations
The timing, location, and method of delivery for pregnancies affected by TTTS depend on the health of both the mother and the twins:
- Timing: Early delivery may be necessary due to obstetrical indications.
- Location: Delivery should occur at a facility equipped with a neonatal intensive care unit (NICU) capable of providing specialized care for premature babies.
- Method: The mode of delivery (vaginal or cesarean section) is determined based on the condition of the twins and maternal health.
Postnatal care and long-term outcomes
At birth, infants will be carefully evaluated for complications. Many are stable and require minimal intervention, allowing immediate bonding with their parents. However, some may need NICU care for prematurity, anemia, or other issues.
Long-term outcomes for TTTS survivors depend on the severity of the disease and the effectiveness of treatment. A multidisciplinary team will guide families in accessing follow-up care, including pediatric specialists, if needed. Neurological complications are monitored closely as they are a potential concern in survivors.
Our outcomes for patients who underwent fetoscopic laser ablation for TTTS
Data includes patients with known delivery/neonatal outcome from September 2011 through October 2024.
Our outcomes and expertise
900+ fetoscopic laser ablations performed for TTTS
73% dual survivor rate
Total Placental Laser Ablations
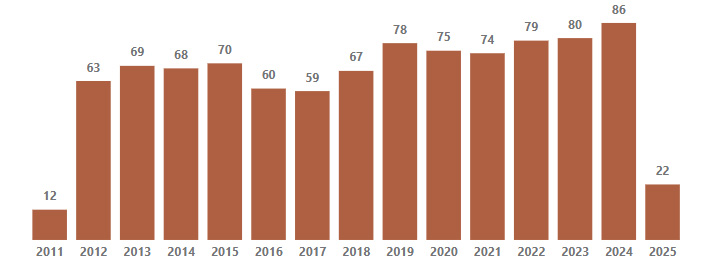
Includes cases from 9/23/2011 to 4/7/2025 (date of Fl procedure);
Includes 9 repeated placental laser ablations.
30-day post-delivery survival rate by TTTS stage at surgery
| TTTS STAGE | DUAL SURVIVORS | SINGLE SURVIVOR | DUAL DEMISE |
|---|---|---|---|
| Stage 1 | 90 (86%) | 12 (12%) | 3 (3%) |
| Stage 2 | 188 (80%) | 24 (10%) | 22 (10%) |
| Stage 3 | 333 (68%) | 98 (20%) | 59 (12%) |
| Stage 4 | 29 (69%) | 9 (21%) | 4 (10%) |
| TTTS plus other diagnoses | 18 (69%) | 5 (19%) | 3 (12%) |
| Overall | 73% | 17% | 10% |
30-day post delivery survival rate by gestational age at surgery
| GESTATIONAL AGE | DUAL SURVIVORS | SINGLE SURVIVOR | DUAL DEMISE |
|---|---|---|---|
| < 18 W 6/7 D | 188 (64%) | 61 (21%) | 43 (15%) |
| 19 W 0/7 D – 21 W 6/7 D | 260 (78%) | 43 (13%) | 30 (9%) |
| 22 W 0/7 D – 24 W 6/7 D | 143 (76%) | 29 (15%) | 16 (9%) |
| 25 W 0/7 D – 27 W | 56 (81%) | 12 (17%) | 1 (1%) |
| 28 W + | 11 (73%) | 3 (20%) | 1 (7%) |
| Overall | 658 (73%) | 148 (17%) | 91 (10%) |
30-day post-delivery survival rate by preoperative cervical length
| CERVICAL LENGTH | DUAL SURVIVORS | SINGLE SURVIVOR | DUAL DEMISE |
|---|---|---|---|
| <1.5cm | 17 (45%) | 8 (21%) | 13 (34%) |
| >=1.5cm | 637 (75%) | 137 (16%) | 78 (9%) |
| Unknown | 4 (57%) | 3 (43%) | |
| Overall | 658 (73%) | 148 (17%) | 91 (10%) |